What is ejemplos de atomos y moleculas?
Ejemplos de atomos y moleculas are building blocks of matter. An atom is the smallest unit of matter that retains all the chemical properties of an element. A molecule is a group of atoms that are held together by chemical bonds. Atoms and molecules are the basic units of chemistry, and they are essential for understanding the structure and properties of matter.
There are many different types of atoms and molecules. The simplest atoms are hydrogen and helium, which consist of one and two protons, respectively. The most complex atoms are the actinides, which have over 100 protons. Molecules can be composed of two or more atoms of the same element, or they can be composed of atoms of different elements. For example, water is a molecule that is composed of two hydrogen atoms and one oxygen atom. Carbon dioxide is a molecule that is composed of one carbon atom and two oxygen atoms.
Atoms and molecules are essential for life. They make up the cells that make up our bodies, and they are responsible for the chemical reactions that occur in our bodies. Atoms and molecules are also essential for the environment. They make up the air we breathe, the water we drink, and the food we eat.
The study of atoms and molecules is called chemistry. Chemistry is a vast and complex field, but it is also a fascinating one. By studying atoms and molecules, we can learn about the fundamental building blocks of matter and the forces that hold them together.
Ejemplos de Atomos y Moleculas
Ejemplos de atomos y moleculas are the basic building blocks of matter. They are essential for understanding the structure and properties of matter, and they play a vital role in many chemical reactions.
- Atoms are the smallest units of matter that retain all the chemical properties of an element.
- Molecules are groups of atoms that are held together by chemical bonds.
- Atoms are composed of a nucleus and electrons.
- Molecules can be composed of two or more atoms of the same element, or they can be composed of atoms of different elements.
- Atoms and molecules are essential for life.
- The study of atoms and molecules is called chemistry.
- Chemistry is a vast and complex field, but it is also a fascinating one.
By studying atoms and molecules, we can learn about the fundamental building blocks of matter and the forces that hold them together. This knowledge is essential for understanding the world around us, and it has led to the development of many important technologies, such as computers, medicines, and fertilizers.
Atoms are the smallest units of matter that retain all the chemical properties of an element.
This statement is a fundamental principle of chemistry. It means that all atoms of a given element have the same chemical properties. For example, all atoms of hydrogen have one proton and one electron, and they all react with oxygen to form water. The chemical properties of an element are determined by the number of protons in the nucleus of its atoms.
- Structure of Atoms
Atoms are composed of a nucleus and electrons. The nucleus is located at the center of the atom and contains protons and neutrons. Protons have a positive charge, while neutrons have no charge. Electrons have a negative charge and orbit the nucleus.
- Isotopes
Atoms of the same element can have different numbers of neutrons. These different forms of the same element are called isotopes. Isotopes have the same number of protons and electrons, but they have different atomic masses.
- Chemical Bonding
Atoms can combine with each other to form molecules. Molecules are held together by chemical bonds. There are different types of chemical bonds, including covalent bonds, ionic bonds, and metallic bonds.
- Chemical Reactions
Atoms can also rearrange themselves to form new molecules. This process is called a chemical reaction. Chemical reactions are essential for life, and they are used to produce many of the products we use every day, such as food, clothing, and medicine.
The study of atoms and molecules is called chemistry. Chemistry is a vast and complex field, but it is also a fascinating one. By studying atoms and molecules, we can learn about the fundamental building blocks of matter and the forces that hold them together. This knowledge is essential for understanding the world around us, and it has led to the development of many important technologies, such as computers, medicines, and fertilizers.
Molecules are groups of atoms that are held together by chemical bonds.
This statement is a fundamental principle of chemistry. It means that molecules are the basic units of matter, and they are composed of atoms that are held together by chemical bonds. The properties of a molecule are determined by the types of atoms that compose it and the way those atoms are bonded together.
- Covalent Bonds
Covalent bonds are formed when two atoms share electrons. This type of bond is the most common type of bond in organic molecules. For example, the molecule methane (CH4) is composed of one carbon atom and four hydrogen atoms. The carbon atom shares its four valence electrons with the four hydrogen atoms, forming four covalent bonds.
- Ionic Bonds
Ionic bonds are formed when one atom transfers electrons to another atom. This type of bond is common in inorganic molecules. For example, the molecule sodium chloride (NaCl) is composed of one sodium atom and one chlorine atom. The sodium atom transfers one electron to the chlorine atom, forming an ionic bond.
- Metallic Bonds
Metallic bonds are formed between metal atoms. This type of bond is common in metals. For example, the metal copper is composed of copper atoms that are held together by metallic bonds.
The formation of molecules is essential for life. Molecules are the building blocks of all living things, and they are responsible for the chemical reactions that occur in our bodies. The study of molecules is called chemistry, and it is a vast and complex field. However, the basic principles of chemistry are relatively simple, and they can be used to explain a wide range of phenomena, from the properties of matter to the reactions that occur in living organisms.
Atoms are composed of a nucleus and electrons.
This statement is a fundamental principle of chemistry. It means that all atoms are composed of a nucleus and electrons. The nucleus is located at the center of the atom and contains protons and neutrons. Protons have a positive charge, while neutrons have no charge. Electrons have a negative charge and orbit the nucleus.
The number of protons in the nucleus determines the element to which the atom belongs. For example, all atoms with one proton are hydrogen atoms. All atoms with two protons are helium atoms, and so on.
The number of electrons in an atom determines its chemical properties. Atoms with the same number of protons but different numbers of electrons are called isotopes. Isotopes have the same atomic number but different mass numbers.
The arrangement of electrons in an atom's electron cloud determines its chemical bonding properties. Atoms with unpaired electrons are more likely to form chemical bonds than atoms with all of their electrons paired.
The study of atoms and molecules is called chemistry. Chemistry is a vast and complex field, but it is also a fascinating one. By studying atoms and molecules, we can learn about the fundamental building blocks of matter and the forces that hold them together. This knowledge is essential for understanding the world around us, and it has led to the development of many important technologies, such as computers, medicines, and fertilizers.
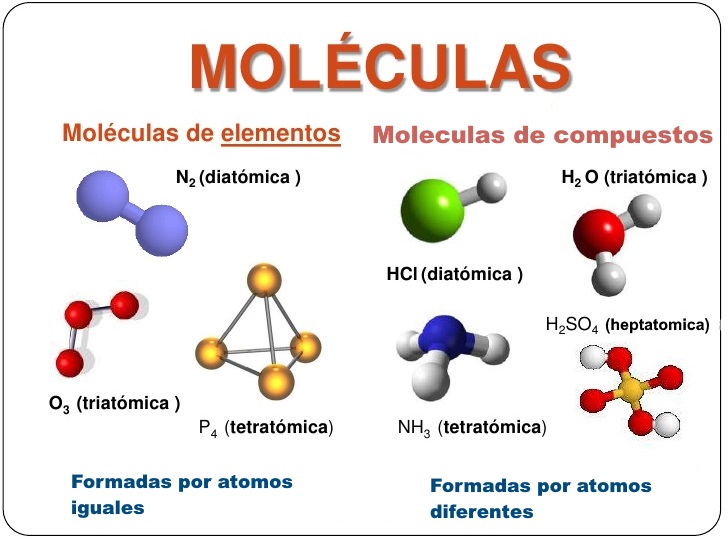
Molecules can be composed of two or more atoms of the same element, or they can be composed of atoms of different elements.
This statement is a fundamental principle of chemistry. It means that molecules, the basic units of matter, can be composed of two or more atoms of the same element, or they can be composed of atoms of different elements. This concept is essential for understanding the structure and properties of matter, and it has a wide range of applications in fields such as medicine, materials science, and energy research.
- Homonuclear molecules
Molecules that are composed of two or more atoms of the same element are called homonuclear molecules. Examples of homonuclear molecules include hydrogen (H2), oxygen (O2), and nitrogen (N2). These molecules are typically nonpolar and have relatively low chemical reactivity.
- Heteronuclear molecules
Molecules that are composed of atoms of different elements are called heteronuclear molecules. Examples of heteronuclear molecules include water (H2O), carbon dioxide (CO2), and methane (CH4). These molecules are typically polar and have higher chemical reactivity than homonuclear molecules.
The composition of molecules has a profound impact on their properties. For example, homonuclear molecules are typically gases at room temperature, while heteronuclear molecules can be gases, liquids, or solids. The chemical reactivity of molecules also depends on their composition. Homonuclear molecules are generally less reactive than heteronuclear molecules because the atoms in homonuclear molecules are more strongly bonded to each other.
The concept of molecules being composed of two or more atoms of the same element, or atoms of different elements, is essential for understanding the structure and properties of matter. This concept has a wide range of applications in fields such as medicine, materials science, and energy research.
Atoms and molecules are essential for life.
Atoms and molecules are the basic building blocks of matter. They make up everything in the universe, from the air we breathe to the food we eat. Atoms are the smallest units of matter that retain all the chemical properties of an element. Molecules are groups of atoms that are held together by chemical bonds.
Atoms and molecules are essential for life because they are the building blocks of all living things. Our bodies are made up of trillions of cells, and each cell is made up of even smaller molecules. These molecules perform a wide range of functions, including providing energy, building and repairing tissues, and transporting oxygen and nutrients throughout the body.
For example, the molecule DNA contains the instructions for building and maintaining an organism. The molecule hemoglobin transports oxygen from the lungs to the rest of the body. And the molecule ATP provides energy for all of the body's cells.
Without atoms and molecules, life as we know it would not be possible. They are the essential building blocks of all living things, and they play a vital role in all of the body's functions.
The study of atoms and molecules is called chemistry.
Chemistry is the branch of physical science that studies the composition, structure, properties, and change of matter. It is a vast and complex field that encompasses many different areas of study, including the study of atoms and molecules.
Atoms and molecules are the basic building blocks of matter. They are the smallest units of matter that retain all the chemical properties of an element. Atoms are composed of a nucleus and electrons, while molecules are groups of atoms that are held together by chemical bonds.
The study of atoms and molecules is essential for understanding the structure and properties of matter. It is also essential for understanding chemical reactions, which are the processes by which atoms and molecules rearrange themselves to form new substances.
Chemistry has a wide range of practical applications, including the development of new materials, drugs, and energy sources. It is also essential for understanding the environment and human health.
The study of atoms and molecules is a fascinating and challenging field. It is a field that is constantly evolving, and new discoveries are being made all the time. If you are interested in learning more about the world around you, then chemistry is a great place to start.
Chemistry is a vast and complex field, but it is also a fascinating one.
The study of chemistry is essential for understanding the world around us. Chemistry is involved in everything from the food we eat to the clothes we wear to the medicines we take. By studying chemistry, we can learn about the composition, structure, properties, and change of matter.
- The vastness and complexity of chemistry
Chemistry is a vast and complex field because there are so many different types of atoms and molecules. There are also many different ways that atoms and molecules can interact with each other. This complexity makes chemistry a challenging but also fascinating field to study.
- The importance of chemistry in everyday life
Chemistry is essential for everyday life. We use chemistry to cook food, clean our clothes, and build our homes. Chemistry is also used to make medicines, plastics, and other materials that we rely on every day.
- The fascinating nature of chemistry
Chemistry is a fascinating field because it is constantly evolving. New discoveries are being made all the time, and our understanding of the world around us is constantly changing. This makes chemistry a field that is always exciting to study.
The study of chemistry is essential for understanding the world around us. Chemistry is involved in everything from the food we eat to the clothes we wear to the medicines we take. By studying chemistry, we can learn about the composition, structure, properties, and change of matter.
FAQs on Atoms and Molecules
This section addresses common questions and misconceptions about atoms and molecules, providing concise and informative answers.
Question 1: What are atoms and molecules?
Atoms are the fundamental units of matter, composed of a nucleus and electrons. Molecules are groups of atoms held together by chemical bonds.
Question 2: How do atoms differ from molecules?
Atoms are individual units, while molecules are combinations of atoms. Molecules have specific structures and properties that arise from the interactions between the constituent atoms.
Question 3: What is the relationship between atoms and elements?
Atoms of the same element have the same number of protons, distinguishing them from atoms of other elements. Elements are classified based on their atomic number, which corresponds to the number of protons in their atoms.
Question 4: How do atoms form molecules?
Atoms combine to form molecules through chemical bonds. These bonds arise from the attraction between positively charged atomic nuclei and negatively charged electrons.
Question 5: What determines the properties of molecules?
The properties of molecules depend on the types of atoms they contain, the number of atoms, and the arrangement of atoms within the molecule.
Question 6: Why are atoms and molecules important?
Atoms and molecules are the building blocks of all matter, and understanding their behavior is crucial for various scientific disciplines, including chemistry, biology, and material science.
Summary: Atoms and molecules are fundamental concepts in chemistry, representing the smallest units of matter and the combinations of atoms that form distinct substances. They determine the properties of matter and play vital roles in chemical reactions and biological processes.
Transition: The exploration of atoms and molecules extends beyond these basic concepts, delving into their interactions, dynamics, and applications in various fields.
Conclusin
A lo largo de este artculo, hemos explorado los conceptos fundamentales de tomos y molculas, reconociendo su importancia como componentes bsicos de la materia. Hemos examinado su estructura, propiedades y el papel crucial que desempean en las reacciones qumicas y los procesos biolgicos.
El estudio de tomos y molculas es esencial para diversas disciplinas cientficas, proporcionando informacin sobre el comportamiento de la materia y facilitando avances en campos como la medicina, la tecnologa de materiales y la investigacin energtica. Al comprender los principios subyacentes que rigen los tomos y las molculas, podemos desbloquear nuevos conocimientos y desarrollar soluciones innovadoras para los desafos que enfrenta nuestra sociedad.
Premium Services For Intermediaries: Enhance Your Business
Conquer I-Ready Reading And Math: A Comprehensive Guide
Awesome Python Collection Class Implementation Example

Definición de Molécula; ejemplos, y estructura (enlaces, y átomos)
Ser vivo Resultante interacción genomaambiente ATOMOS Y MOLECULAS

King's College London Faculty of Natural & Mathematical Sciences